Lenoir, NC May 31, 2019 – Exela Pharma Sciences is pleased to announce the approval and immediate launch of ELCYS™ (cysteine hydrochloride injection), USP, the only FDA Approved cysteine hydrochloride injection on the market in the United States. ELCYS™ was approved under priority review as a New Drug Application (NDA). It is not an Abbreviated New Drug Application (ANDA) product. The NDA is the result of significant research and development aimed at lowering the Aluminum content in the drug product. ELCYS™ is labeled to contain no more than 120 parts per billion (mcg/L) of Aluminum. ELCYS™ is indicated for use
Read More
Exela CEO Dr. Phanesh Koneru Interviews President George W. Bush at Becker’s Healthcare Conference
April 13, 2018 | Chicago, Illinois On Friday, April 13, 2018, Phanesh Koneru, PhD, LLM, President and CEO of Exela Pharma Sciences interviewed the Honorable George W. Bush, 43rd President of the United States of America at the 9th Annual Becker’s Hospital Review conference in front of 4,000 attendees. The conference is attended by the nation’s leading healthcare CEOs, CFOs and hospital personnel responsible for decision making in America’s hospital networks. President Bush was the Keynote Speaker at Friday’s meeting. During the one hour of highly entertaining and informative interview, Dr. Koneru explored many aspects of the Bush Presidency, including
Read More
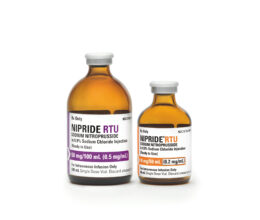
EXELA Pharma Sciences, LLC Receives Approval for NIPRIDE® RTU, (sodium nitroprusside) in 0.9% sodium chloride injection, 10mg / 50mL (200 mcg/mL), the First Ready to Use 200 mcg/mL sodium nitroprusside injection.
Lenoir, NC February 9, 2018 – Exela Pharma Sciences, LLC (“Exela”) today announced that it received FDA approval for NIPRIDE® RTU (sodium nitroprusside) in 0.9% sodium chloride injection, 10mg/50mL (200 mcg/mL) as a supplement to the currently marketed NIPRIDE® RTU 50mg/100mL. NIPRIDE® RTU (sodium nitroprusside) is indicated for the immediate reduction of blood pressure of adult and pediatric patients. NIPRIDE® RTU is also indicated for producing controlled hypotension to reduce bleeding during surgery. NIPRIDE® RTU is also indicated for the treatment of acute heart failure to reduce left ventricular end-diastolic pressure, pulmonary capillary wedge pressure, peripheral vascular resistance and mean
Read More
EXELA Pharma Sciences, LLC Begins Shipments of Ganciclovir Injection in 0.8% sodium chloride solution, the First Ready to Use Ganciclovir Injection
Lenoir, NC September 18, 2017 – Exela Pharma Sciences today announced that it began shipments of its FDA approved Ganciclovir Injection in 0.8% sodium chloride solution on September 18, 2017 to wholesalers across the country. Ganciclovir Injection is indicated for the treatment of cytomegalovirus (CMV) retinitis in immunocompromised adult patients, including patients with acquired immunodeficiency syndrome (AIDS) and for the prevention of CMV disease in adult transplant recipients at risk for CMV disease. Ganciclovir carries a boxed warning. Please see safety information below and additional important safety information in the full prescribing information by clicking here. Exela’s Ganciclovir Injection is
Read More
EXELA Pharma Sciences, LLC Wins 2017 Manufacturing Leadership Award
WINSTON-SALEM, NC – The North Carolina Manufacturing Extension Partnership (NCMEP), a public-private operating alliance working to help manufacturing companies become operationally efficient and well positioned to grow profitably, today announced the winners of its 2017 Manufacturing Leadership Awards at mfgCON in Winston-Salem, North Carolina. The state awards program recognizes manufacturers for their commitment to the North Carolina manufacturing sector, as proven by outstanding performance in the areas of Innovation, Sustainable Manufacturing, Advanced Talent Development, Developing Markets and Continuous Improvement. “Our state’s manufacturers continue to expand and excel in performance, leading to sustained customer satisfaction,” says Phil Mintz, NCMEP Director and Executive
Read More
IMPORTANT DRUG WARNING
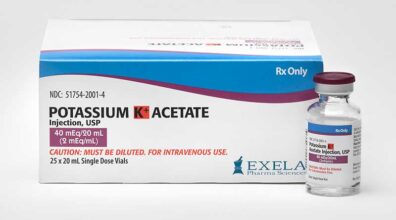
Dear Health Care Provider: Re: RISK OF POTENTIAL ALUMINUM TOXICITY WITH USE OF POTASSIUM ACETATE 40 MEQ/20 ML INJECTION PARTICULARLY IN NEONATAL PATIENTS AND PATIENTS WITH RENAL IMPAIRMENT The purpose of this letter is to inform you of important safety information for Potassium Acetate 40 meq/20 mL (2 meq/mL) Injection (NDC 51754-2001-4), manufactured and supplied by Exela Pharma Sciences, LLC (“Exela”). The current carton and vial labels for Exela’s Potassium Acetate 40 meq/20 mL (2 meq/mL) Injection indicate the product contains an aluminum level of not more than 200 mcg/L. We have determined that the actual aluminum level in some instances
Read More
EXELA Pharma Sciences, LLC Receives Approval for Ganciclovir Injection in 0.8% sodium chloride solution, the First Ready to Use Ganciclovir Injection
Lenoir, NC February 17, 2017 – Exela Pharma Sciences today announced that it received FDA approval for Ganciclovir Injection in 0.8% sodium chloride solution. Ganciclovir Injection is indicated for the treatment of cytomegalovirus (CMV) retinitis in immunocompromised adult patients, including patients with acquired immunodeficiency syndrome (AIDS) and for the prevention of CMV disease in adult transplant recipients at risk for CMV disease. Ganciclovir carries a boxed warning. Please see safety information below and additional important safety information in the full prescribing information by clicking here. Exela’s Ganciclovir Injection is the first ganciclovir product available in a ready to use formulation.
Read More
EXELA Pharma Sciences, LLC Receives Approval for NIPRIDE RTU, (sodium nitroprusside) in 0.9% sodium chloride injection, the First Ready to Use sodium nitroprusside injection.
Lenoir, NC March 9, 2017 – Exela Pharma Sciences today announced that it received FDA approval for NIPRIDE RTU (sodium nitroprusside) in 0.9% sodium chloride injection, the company’s second FDA approval this year. NIPRIDE RTU (sodium nitroprusside) is indicated for the immediate reduction of blood pressure of adult and pediatric patients. NIPRIDE RTU is also indicated for producing controlled hypotension to reduce bleeding during surgery. NIPRIDE RTU is also indicated for the treatment of acute heart failure to reduce left ventricular end-diastolic pressure, pulmonary capillary wedge pressure, peripheral vascular resistance and mean arterial blood pressure. NIPRIDE RTU carries a boxed
Read More
EXELA Pharma Sciences Receives Supplier Horizon Award from Premier Inc.
Lenoir, NC July 5, 2016 – Exela Pharma Sciences today announced that it is the winner of 2016 Supplier Horizon Award in Pharmaceutical Services and Technology by Premier, Inc. (NASDAQ: PINC). At a ceremony hosted by Premier in National Harbor, MD, Exela was recognized for its support of Premier members through exceptional local customer service and engagement, value creation through clinical excellence and commitment to lower costs. Mark Hartman, Exela’s Chief Commercial Officer, accepted the award. “It is a great honor to receive the Horizon Award from Premier, one of the leading healthcare improvement and transformation companies in our industry.
Read More
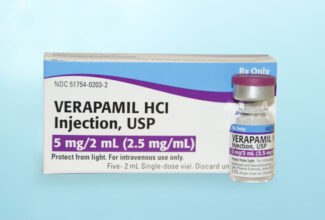
Exela Pharma Sciences Announces Availability of Verapamil HCl Injection, USP VIALS
Exela Pharma Sciences today confirmed the approval of its New drug Application (NDA) and availability of Verapamil HCl Injection, USP 5mg vials (2.5mg/ml). Product is stocked in the major wholesaler distribution channels, and is available in a convenient carton of 5 vials each. This lower pack size of 5 vials coupled with affordable contract pricing available through local Group Purchasing Organizations, leads to significant savings and lower inventory costs for hospitals and healthcare providers. The Verapamil HCl 5mg Injection vials also offer the convenience and safety of a vial versus an ampule. Exela’s Verapamil HCl Injection carries an AP rating
Read More

 888-451-4321
888-451-4321