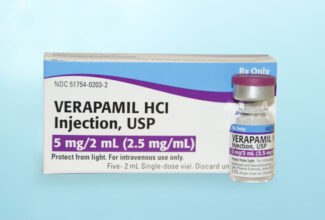
Exela Pharma Sciences today confirmed the approval of its New drug Application (NDA) and availability of Verapamil HCl Injection, USP 5mg vials (2.5mg/ml). Product is stocked in the major wholesaler distribution channels, and is available in a convenient carton of 5 vials each. This lower pack size of 5 vials coupled with affordable contract pricing available through local Group Purchasing Organizations, leads to significant savings and lower inventory costs for hospitals and healthcare providers. The Verapamil HCl 5mg Injection vials also offer the convenience and safety of a vial versus an ampule. Exela’s Verapamil HCl Injection carries an AP rating in the FDA’s Orange Book listing.
Exela proudly manufactures its FDA approved NDA and Abbreviated New Drug Application (ANDA) products at its sterile manufacturing site in Lenoir, North Carolina, USA. Verapamil HCl Injection marks the fourth sterile injectable product that Exela has launched in the US market over the past year under its own Exela label, joining Caffeine Citrate Injection, Caffeine Citrate Oral Solution and Potassium Acetate Injection.
Phanesh Koneru, Ph.D., LL.M., CEO and President of Exela Pharma Sciences, stated: “Exela’s introduction of Verapamil HCl Injection vials into the market fills a critical need at hospitals trying to lower their overall drug spend and is consistent with our Mission statement to provide access to high quality affordable medicines. Our US based sterile manufacturing site has an excellent compliance record with the FDA and is a reflection of our commitment to excellence in quality and overall commitment to supply chain fulfillment responsibility. We are excited about the opportunity to serve the market needs for this product and many others to come in the near future.”
About Exela Pharma Sciences
Exela Pharma Sciences, headquartered in Lenoir, North Carolina, is a privately held specialty pharmaceutical company that was founded in 2005 to develop, manufacture and market high quality ANDA and NDA products in the sterile injectable and ophthalmic arena. The company has rapidly grown from research and development stage through contract manufacturing to a fully integrated specialty pharmaceutical company with a deep pipeline of ANDA and specialty 505(b)(2) products. The company employs over 185 people in its Lenoir, North Carolina operations. Exela currently manufactures and supplies a number of products through its partners in the US, including Nicardipine Injection, Ibuprofen-Lysine Injection, Magnesium Sulfate Injection, Clonidine Injection, Paricalcitol Injection and Argatroban Injection.

 888-451-4321
888-451-4321