CDMO Services
Proven Track Record of Execution Under Intense Time Pressures with Exceptional Service
- Manufacturing
- Analytics
- Inspection, Labeling and Packaging
- Warehousing, Storage and Distribution
Exela Pharma Sciences, located one hour west of Charlotte Douglas International Airport in North Carolina, is a full-service contract development and manufacturing organization – CDMO. With over 320,000 sqft of R&D, cGMP, warehousing, storage and distribution services spread over four facilities (and an additional 200,000 sqft under development), Exela is capable and ready to address pharmaceutical and biotech industry needs from early development through clinical materials and commercial supply of tens of millions of units.
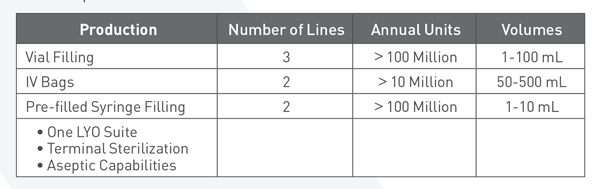
Manufacturing
There’s never been a more demanding time for filling of sterile injectables, including biologics, orphan drugs and personalized medicines. Choosing the right drug product development and manufacturing partner can help speed up the design, build and validation process – ultimately accelerating market entry. Exela has a proven track record of execution under intense time pressures with exceptional service

Analytical and Microbiology
Exela is committed to quality and scientific excellence. With in-house fully equipped analytical and microbiology laboratories, we address most of product analytical/stability needs expeditiously. Examples include, Impurity Identification, Extractable & Leachable Studies, ISO Syringe Testing and Sterility Testing (with isolator) with species identification.
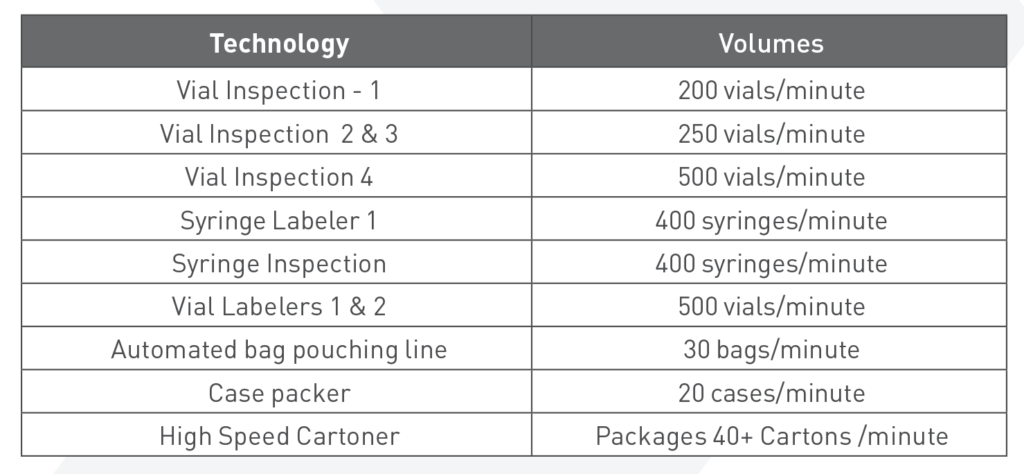
Inspection, Labeling and Packaging
Our state-of-the-art 117,000 square foot Elma facility is our downstream finishing center of excellence with six automated inspection, labeling and packaging lines. We are capable of addressing packaging needs from clinical trial supplies to commercial manufacturing of tens of millions of units.
Warehousing, Storage and Distribution
Exela has several thousand GMP pallet spaces for ambient temperature storage. We currently have freezer farm capabilities for -80C, and we are building -20c and 2-8C storage capabilities. We also have dry ice production and packaging capabilities. Exela is fully licensed by the DEA to handle C-II through C-V and listed drugs. In addition, Exela has its own ordering portal to take orders and ship directly to customers in the majority of the states.

 888-451-4321
888-451-4321